Pipeline Program
ANN-01M
ANN-01M is a bispecific antibody in pre-clinical development for BCMA-positive multiple myeloma.
Multiple Myeloma Disease Landscape
Multiple myeloma is a malignant plasma cell disorder that accounts for ~10% of hematologic cancers and ~2% of all cancers. The American Cancer Society estimates ~36,110 new U.S. diagnoses and ~12,030 deaths in 2025—highlighting substantial unmet need despite progress with proteasome inhibitors, IMiDs, anti-CD38 mAbs, and BCMA-directed therapies.
Patients typically experience cycles of remission and relapse, cumulative toxicity, and end-organ damage (bone, renal). Outcomes are especially poor in triple-class-refractory disease, where survival can be measured in months. Differentiated, off-the-shelf mechanisms that do not depend on T-cell fitness remain a priority for later-line care.
Incidence Snapshot
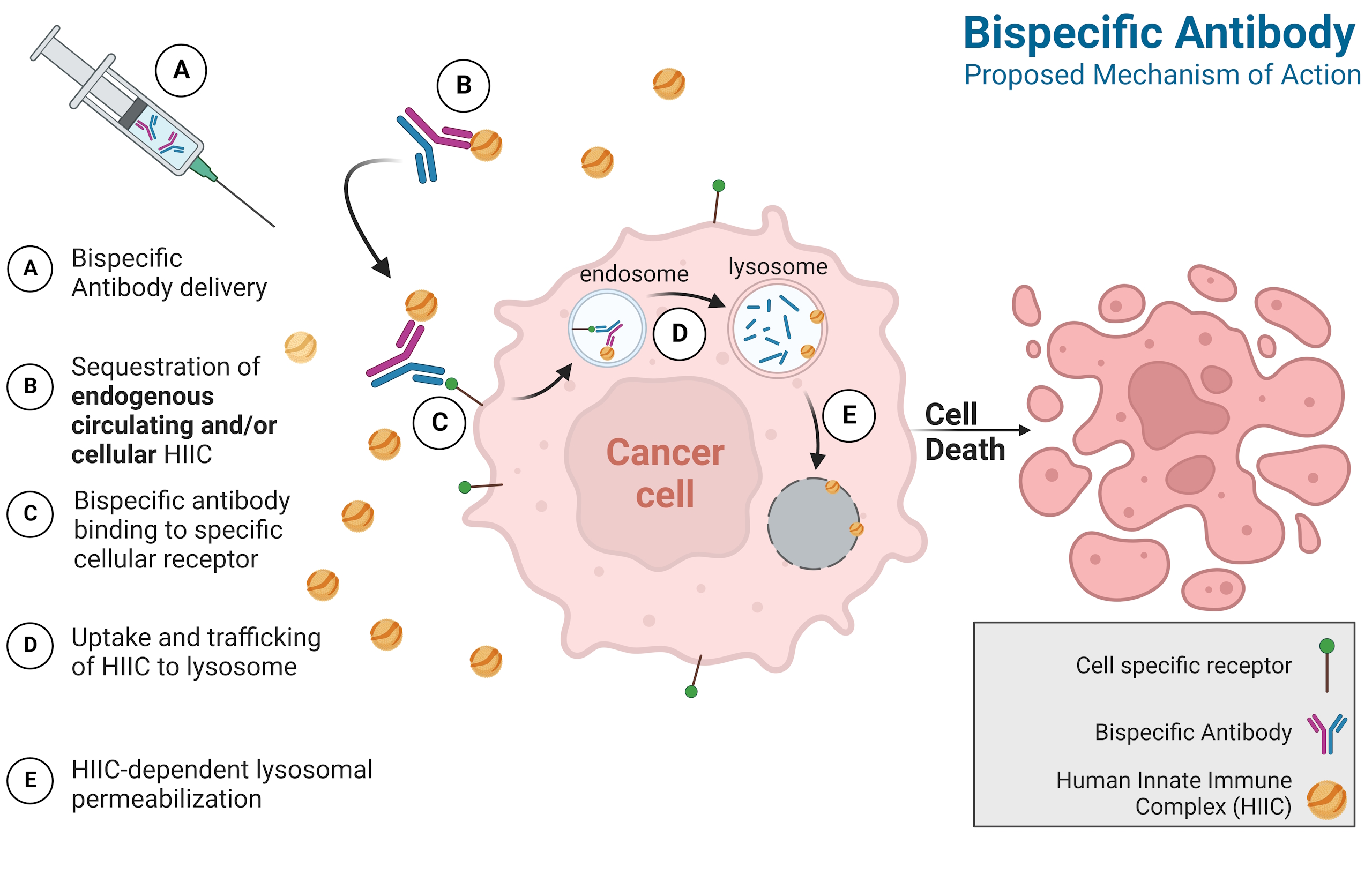
BCMA as a Precision Anchor
BCMA (B-cell maturation antigen) is found on mature B cells and plasma cells, where it promotes survival signals that myeloma cells exploit.
While BCMA-directed CAR-T and bispecific therapies have proven the target’s value, they face challenges like T-cell exhaustion and limited accessibility. ANN-01M offers an immune-independent approach that preserves BCMA as a target while reducing systemic toxicity and simplifying development.
ANN-01M Program & Research Plan
Mechanism & Rationale
- ANN-01M bispecific escorts endogenous ApoL1 into BCMA+ myeloma cells; post-internalization lysosomal pore formation delivers immune-cell–independent killing.
- Designed as an off-the-shelf, redosable option suitable for heavily pre-treated, immunosuppressed patients.
Current Progress (pre-clinical)
- In-vitro: BCMA-dependent binding, rapid internalization, ApoL1-redirected killing in BCMA+ lines; no activity in BCMA-negative controls.
- Selectivity & Safety: Early off-target screens;
Translational Models (in flight / planned)
- Emory/Winship access to myeloma PDX and primary samples for benchmark comparisons.
- Transgenic Human ApoL1-capable NSG mouse models to assess efficacy and safety in a relevant background.
- γ-secretase inhibitor in-vivo studies to evaluate mBCMA vs sBCMA effects on exposure and response.
Regulatory Workstreams
- Focused on MoA validation, early tox signals, expanded animal model choices - gates supporting an INTERACT → pre-IND path.
- CMC readiness: sequence/format lock, pilot expression, developability & stability screens, and CDMO down-selection for GLP tox material; business development for future GMP capacity.
Near-Term Milestones
- Complete expanded in-vitro panel and BCMA threshold analysis; initiate PDX efficacy.
- Finalize tox strategy in humanized/NSG models; progress IND-enabling study design.
- Deliver CMC feasibility package (titre/yield, HCP/HC DNA analytics, stability screens).
