Our approach for targeting cancer
Approach
Harnessing the Power of Innate Immunity
Redirecting ApoL1 to Defeat Tumor Resistance
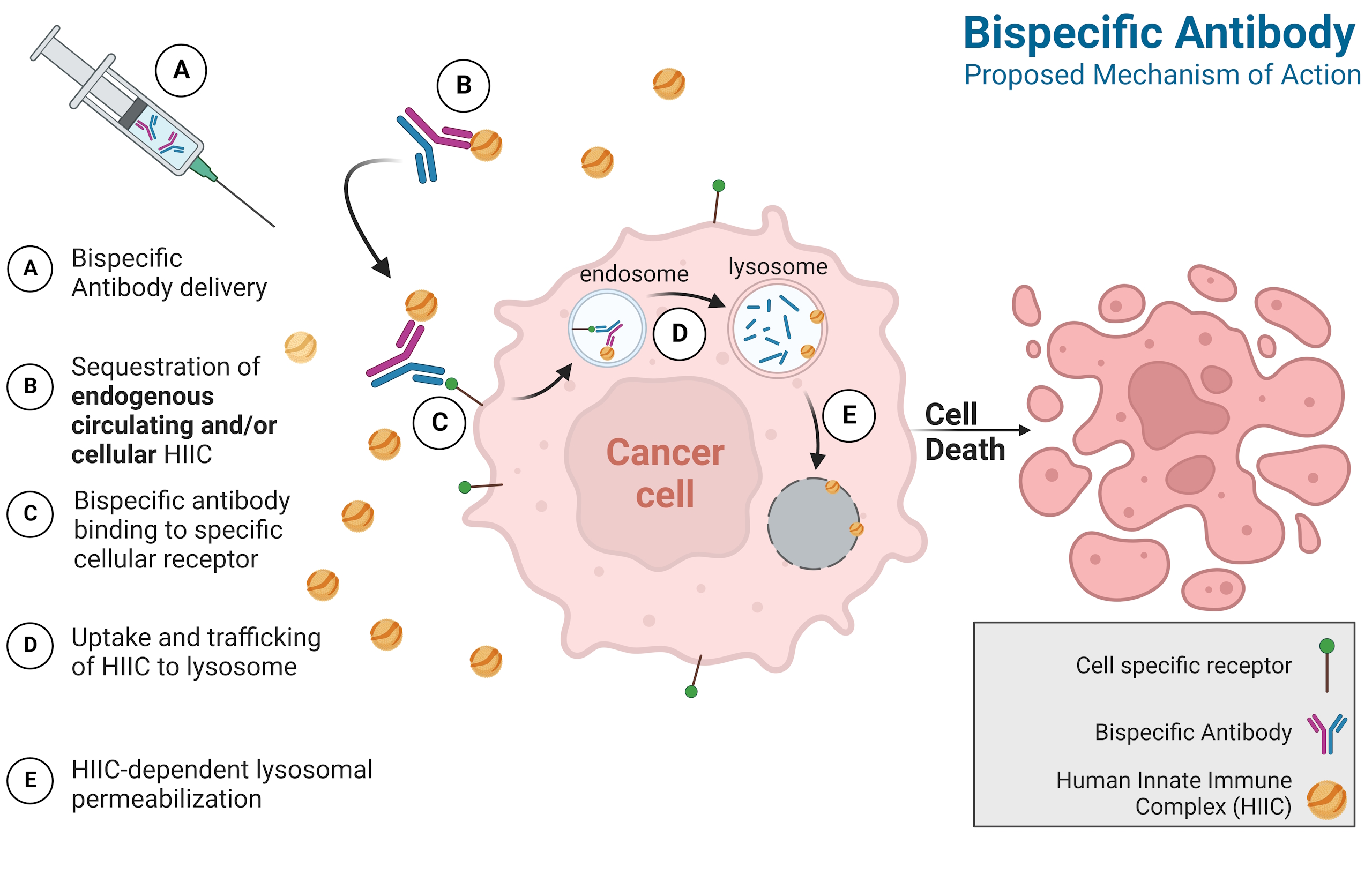
Annate Bitherapeutics develops bispecific antibodies that deliver ApoL1—a potent component of the body's innate immune defense—directly into cancer cells. This unique approach enables immune-independent tumor killing that complements and extends today's cancer therapies
- Targets cancer cells with precision using the body's own proteins
- Bypasses T-cell exhaustion and immune escape
Founders Spotlight
Hear how Annate Bitherapeutics is translating innate immunity into precise oncology therapeutics.
Our Platform In Brief
By co-opting ApoL1's native ability to disrupt membranes, The Annate Platform is designed to transport innate immune complexes past the tumor surface and into lysosomal compartments. Once inside, ApoL1 destabilizes the lysosome and triggers cell death without relying on effector cells.
Native Biology As A Payload
We harness naturally circulating ApoL1 within high-density lipoprotein particles (HDLs), maintaining its native conformation and reducing the potential for immunogenicity.
Bispecific Targeting
Engineered antibody arms selectively bind tumor-associated surface antigens, directing ApoL1 deposition exclusively to sites of disease.
Immune-System Agnostic
Because tumor cell killing is mediated by harnessing circulating ApoL1 within HDL particles, the Annate Platform remains effective even under conditions of immune suppression or T-cell exhaustion.
How The Annate Platform Works
Our adaptable discovery platform shortens the path from target to candidate, generating powerful translational insights at every step.
- Target Discovery
We identify overexpressed antigens with limited normal-tissue distribution.
- Antibody Engineering
Our bispecific antibodies are precisely designed to balance strong binding with efficient uptake into tumor cells.
- Translational Readiness
We validate each candidate using functional assays, cell-based studies, and patient-derived models to ensure targeted delivery and potent activity.
- Combination Strategy
We evaluate how our therapeutics can enhance or complement existing standards of care.
Why This Matters
Lysosomal disruption is a novel mode of action that complements existing standards and may overcome cross-resistance.
Payload is endogenous and only unleashed inside targeted cells, maintaining a wide therapeutic window.
Many relapsed patients have exhausted T-cell-dependent options. The Annate Platform cell-intrinsic approach keeps working when lymphocyte counts fall.
